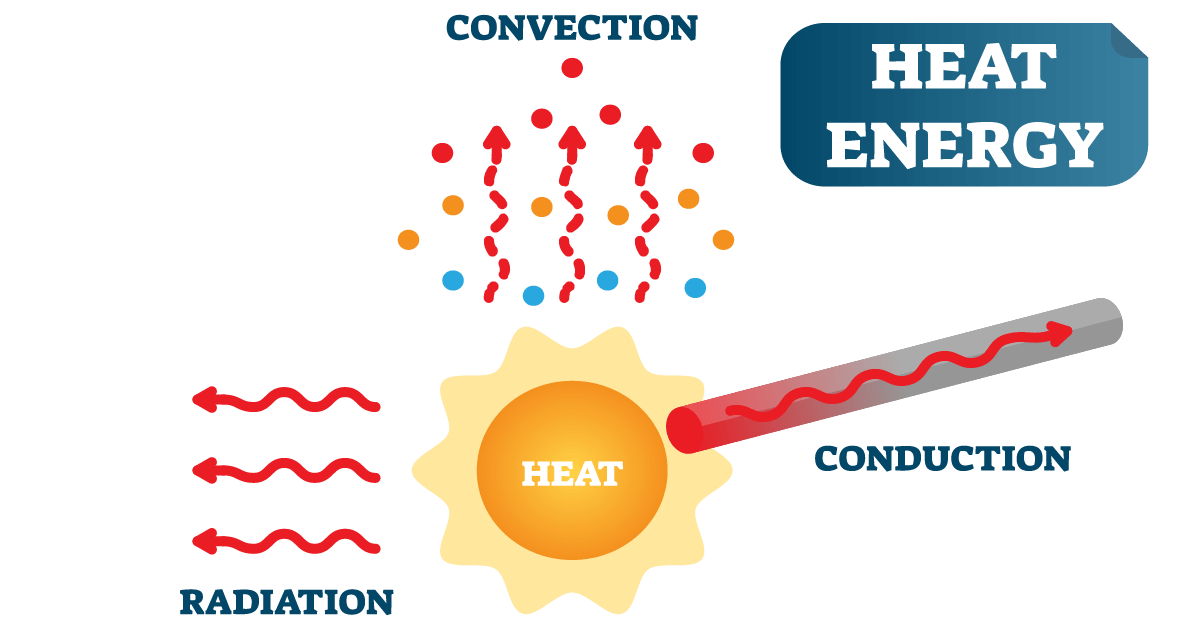
Heat transfer occurs in four ways: convection, conduction, radiation, and phase change. Conduction occurs when atoms or molecules interact with each other. It happens most frequently in solids but also occurs in liquids and gases.
Unlike convection, it does not involve a bulk movement of molecules. Instead, it occurs due to molecular agitation and energy transfer through a solid material.
When a substance increases in temperature, the atoms or molecules gain kinetic energy and vibrate faster. When these particles collide, thermal energy transfers from the higher energy particle to the lower energy one. The cooler molecules begin to vibrate more after they encounter the warmer, more energized molecules.
This process continues from the warmer area of the material to the cooler area. Heat is transmitted through the substance until it reaches thermal equilibrium. For conductive heat transfer to occur between molecules, they must be in close contact.
What is thermal conductivity?
Thermal conductivity is a measurement of a material’s ability to transfer heat energy. Thermal energy transfers from areas of high temperature and molecular agitation to regions of low temperature and agitation. This process occurs until the material reaches a uniform temperature.
The rate of heat flow depends on the magnitude of the temperature gradient and the specific thermal characteristics of the material. The equation for the rate of conductive heat flow is based on Fourier’s law.
Thermal conductivity is the reciprocal of thermal resistivity.
How thermal conductivity varies
Conductive heat transfer varies according to several factors. These include:
- Temperature gradient
- The material’s specific properties
- The length of the path of heat travel
Different materials transfer heat at different rates. For example, air has low conductivity, while metals like copper have high conductivity. These differences inform the design of many materials for everyday use.
Engineers often use materials that are particularly poor conductors to reduce the flow of heat or electricity. These materials are called insulators.
Heat transfer and structure
The structure of a material influences the rate of heat transfer within it. For conductive heat transfer, materials are divided into three categories. These categories are:
1. Metallic solids
Metallic solids, except for graphene, are high electrical and heat conductors. The same molecules within the material transfer both types of energy. However, temperature influences these properties. Temperature and thermal conductivity are positively correlated, while temperature and electrical conductivity are negatively correlated.
2. Non-metallic solids
The molecules in non-metallic solids are arranged into lattices. The vibration of molecules within these substances causes thermal transfer. The closer proximity of the molecules means that non-metallic solids are better conductors; however, variation exists between these materials.
3. Gases
Gases have low thermal conductivity because their molecules rarely come into close contact with each other.
The influence of temperature
The material’s temperature influences the rate of conductive heat transfer. It can change drastically as the temperature increases or decreases. Excited molecules move faster and transfer energy more efficiently than cold and unexcited molecules.
The hot take
Heat transfer is a critical component of the relationship between different materials. Understanding it is crucial for the design of thermal systems across industries. It influences the design of both conductors (such as in the field of electronics) and insulators. Engineers use thermal insulators to reduce heat loss and transfer.